What is NHS research?
Research refers to systematic investigations used to generate new knowledge. Research conducted within the NHS is vital for providing the evidence needed to improve treatments and outcomes for patients. Research can help:
- Better understand different diagnoses
-
Improve NHS treatments
-
Discover treatments that work well
-
Find new treatments
-
Answer questions that are important for patients, service users and carers
Types of clinical research
Clinical research means the study of people’s health and behaviour. This provides the foundation of healthcare, through making sure that treatments are safe and effective.
Clinical research can take many forms; however, these can be divided into two main groups:
-
Interventional studies (clinical trials)
These studies involve making a change to a treatment to determine the outcome. For example:
- Testing a medication
- Testing a psychological therapy
- Observational studies
These studies involve improving understanding of a situation without making changes to treatment. For example:
- Completing a survey
- Providing a blood, urine or saliva sample
What our department do
Our department supports interventional and observational research. This provides the evidence that researchers need to understand how to better support people experiencing mental health problems, neurodiverse diagnoses, substance misuse, and dementia.
Our Take part in research page provides an overview of the research studies we are currently supporting.
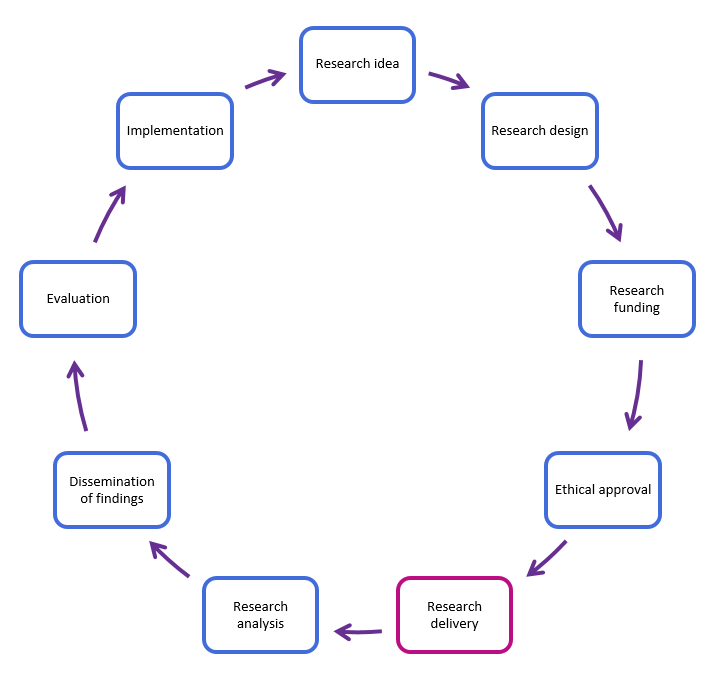
The research cycle shows the different steps of conducting a research study, from developing an idea to implementing research findings.
Our department delivers NHS research, supported by the National Institute for Health and Care research (NIHR) and ethically approved by the Health Research Authority (HRA).
In practice, this means our staff mainly support AWP service users, carers and staff to access relevant NIHR research opportunities offered through AWP. The role of our Research Practitioners and Nurses includes, but is not limited to, the following:
- Providing AWP service users, carers or staff who could be eligible to take part in a specific research study with relevant information
- Making sure that those who are eligible, suitable and want to take part in a research study are fully informed. This means people interested in taking part in research have an understanding of what taking part in a research study would by like for them and have opportunities to ask questions
- Receiving and documenting informed consent
- Supporting those taking part in research with completing questionnaires and surveys
- Checking physical observations such as blood pressure, weight etc.
- Taking blood, urine or saliva samples
- Completing assessments with people at the beginning and throughout a study
- Organising and arranging appointments with research doctors and therapists for those taking part in studies
What is the Difference Between Research and...?
Service Evaluations are designed to judge or compare the worth of a service.
There are different types of evaluations. Evaluations can focus on implementation and learning, how a service works or whether a service change has worked.
The key differences between Service Evaluations and Research are:
| Research | Service Evaluation | |
|---|---|---|
| Goal |
Research may not always have a clear goal, but will seek to gain new knowledge about a subject |
Judge or define current care or service to improve it or understand why some services work well |
| Scope | Research can be conducted by small teams up to several organisations working together. The study participants are only a small population of the wider group a treatment or service is looking to improve the outcomes of | Service evaluations are conducted by service providers and should be registered with the QI and Clinical Audit Team. They can be small or large scale depending on the service. |
| Benefit | The organisations running a study may not receive any direct benefits as research looks to improve individuals or groups outcomes. | The service and service users benefit from service evaluations. Service evaluations looks to check what is working, what is not and where improvements can be made to better care |
| Design | A research project follows the scientific method of generating a hypothesis and working to prove or disprove it by gathering data and analysing the results and publishing results | Service evaluations follow the evaluation cycle. It seeks to answer key questions about the quality of care provided. Results and recommendations are given to stakeholders to inform decision-making. |
| Funding | Research projects funding is usually from an external company or organisation than the one running the study. | Service evaluations are funded by the service and its stakeholders. |
A clinical audit is a way to assess if a healthcare service is being provided in line with the current clinical standards. It lets care providers and patients know what elements of their service that are doing well and where there could be improvements. It is a continual process, where improvements and changes are re-audited to assess the impact of these changes and if there could still be improvements made.
The key differences between Clinical Audits and Research are:
| Research | Clinical Audit | |
|---|---|---|
| Goal | Research may not always have a clear goal, but will seek to gain new knowledge about a subject | Designed and conducted to produce information to inform delivery of best care. Asks if a service is meeting its predetermined or established clinical standards. |
| Scope | Research can be conducted by small teams up to several organisations working together. The study participants are only a small population of the wider group a treatment or service is looking to improve the outcomes of | Audits are conducted by service providers and the QI and Clinical Audit Team. Service users may provide Feedback on the service's quality of care but are not study participants. |
| Benefit | The organisations running a study may not receive any direct benefits as research looks to improve individuals or groups outcomes. | The service and its users benefit from an audit as it is a continual process to meet or exceed current clinical standards. |
| Design | A research project follows the scientific method of generating a hypothesis and working to prove or disprove it by gathering data and analysing the results. | Audits follow the clinical audit cycle. A baseline standard is compared to the current service, recommendations are made, and changes are implemented. Changes are then re-audited. |
| Funding | Research projects funding is usually from an external company or organisation than the one running the study. | Audits are funded by the NHS and its services. |
Quality improvement (QI) is a systematic process to continually improve healthcare services, with a focus on repetitive change, learning and adaptation. QI emphasises a multidisciplinary approach to problem solving, and focuses not on individuals, but on systems of patient care which might be the cause of variations.
Key differences between QI and Research:
| Research | Quality Improvement | |
|---|---|---|
| Goal | Research may not always have a clear goal, but will seek to gain new knowledge about a subject | QI has the goal to optimise processes or improve healthcare outcomes within an organisation |
| Scope | Research can be conducted by small teams up to several organisations working together. The study participants are only a small population of the wider group a treatment or service is looking to improve the outcomes of | QI is conducted within the organisation. Everyone within the scope of the QI of a service will be part of the QI process and affected by the results. QI is supported by the QI and Clinical Audit Team. |
| Benefit | The organisations running a study may not receive any direct benefits as research looks to improve individuals or groups outcomes. | QI provides a direct benefit to the organisation. It will give a desirable outcome to the organisation such as improved care, productivity or efficiency |
| Design | A research project follows the scientific method of generating a hypothesis and working to prove or disprove it by gathering data and analysing the results. | QI uses Improvement Science which is an emerging field of study focused on the methods, theories and approaches that facilitate efforts to improve quality. It frequently uses small datasets, measurement strategies and tools to understand whether variation is normal or if a change made on a given process or activity had the desired impact. |
| Funding | Research projects funding is usually from an external company or organisation than the one running the study. | QI's are typically funded internally by the organisation running them. |
Please note that we do not conduct service evaluations, clinical audit, Quality Improvement projects. Our department are also not responsible for information and data analysis. Our main role is to support AWP service users, carers and staff taking part in NIHR research opportunities being offered within the Trust.
Guidance for your own research
The NHS Health Research Authority has created a tool to see if your study would be considered research according to the UK Policy Framework for Health and Social Care Research guidelines and based on information provided by the Health Research Authority (HRA) Research Ethics Service. Please note that research conducted within the NHS requires HRA ethical approval.
Click the button below to view the decision tool.
Further research resources are listed below.
NIHR Researchers page: Features advice and support on running your own study.
Study Support Service: Assist Researchers and the life sciences industry to plan, set up and deliver research in the NHS and the wider health and social care environment, across England.
Research Capability Funding: Explains how the National Institute for Health and Care Research (NIHR) can fund NHS Trusts to undertake their own research and studies.